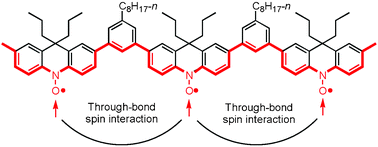
A stable polyradical carrying aminoxyls in the π-conjugated main chain was synthesized and its through-bond spin interaction was investigated. The polyradical is constructed with 1,3-phenylene as a spin coupler and 9,9-di-n-propyl-9,10-dihydroacridin-10-yloxy as a stable spin source. The synthetic method of the spin source moiety was modified by using conventional organic reactions from a previously reported method. The polymeric structure was synthesized by Suzuki–Miyaura coupling. The degree of polymerization and the spin concentration of the polyradical were ca. 11 and 0.71 spins per repeating unit, respectively, indicating that there were ca. 8 spins per molecule. The average S value of the polyradical was 3/2, therefore, 3 spins out of 8 spins were aligned in the polyradical. This showed that the through-bond spin interaction occurs more effectively than in our previous polyradical carrying aminoxyls in the side chain.