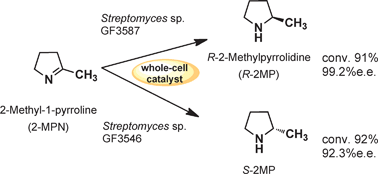
Streptomyces sp. GF3587 and 3546 were found to be imine-reducing strains with high R- and S-selectivity by screening using 2-methyl-1-pyrroline (2-MPN). Their whole-cell catalysts produced 91 mM R-2-methylpyrrolidine (R-2-MP) with 99.2%e.e. and 27.5 mM S-2-MP (92.3%e.e.) from 2-MPN at 91–92% conversion in the presence of glucose, respectively.