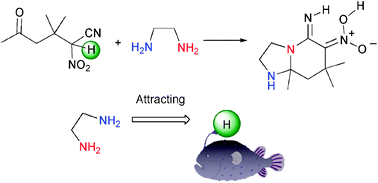
α-Nitro-δ-keto nitriles and α-nitro-δ-keto ester were readily converted to diazabicyclo compounds having vicinal functionality upon treatment with diamines. The keto nitrile attracts the diamine nearby to an acidic hydrogen to cause the pseudo-intramolecular imination which proceeds efficiently without any catalyst at room temperature.