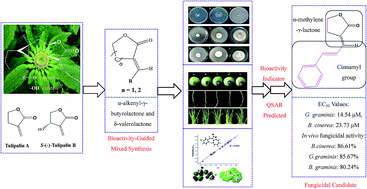
In view of the great antifungal activities of sesquiterpene lactones and natural product Tulipalin A, 52 derivatives derived from α-methylene-γ-butyrolactone substructures were synthesized to study antifungal activities. In vitro and in vivo antifungal activity results revealed that compounds 2-25, which contain a γ-butyrolactone scaffold and cinnamic aldehyde moiety, have greater potent fungicidal activity than other compounds. The preliminary structure–activity relationships (SARs) demonstrated that compounds with electron-withdrawing groups and small steric hindrance would have more desirable potency. Meanwhile, the quantitative structure–activity relationship (QSAR) model (R2 = 0.947, F = 65.77, and S2 = 0.0028) revealed a convincing correlation of antifungal activity against B. cinerea with molecular structures of title compounds. The present study provided a more detailed insight into the antifungal activity of the α-methylene-γ-butyrolactone substructure, which provided a potential expectation for the exploration of α-alkenyl-γ-butyrolactone structures in agriculture.