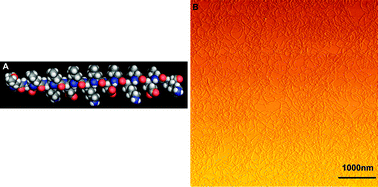
A newly designed self-assembling peptide, P4 (Ac-NH-LDLKLELKLDLKLELK-CONH2), capable of stabilizing hydrophobic compounds in aqueous solution has been discovered. The ionic self-complementary peptide P4 has 16 amino acids, ∼5 nm in size, with an alternating polar and non-polar pattern. Circular dichroism analysis demonstrated that P4 forms stable β-sheet structures, and self-assembles into nanofibers, which was demonstrated by atomic force microscopy. These nanofibers can form a scaffold hydrogel consisting of >99% water. It showed that the P4 hydrogel had stable hydrogel rheological properties. The ability of the peptide to stabilize the hydrophobic anticancer agent ellipticine was tested in this research. The results showed that the state of ellipticine in the complexes was dependent on the concentration of the peptide, which also affected the size and morphology of the complex. The anticancer activity of the complexes was studied by testing the viability with a MTT assay and a LIVE/DEAD® Viability/Cytotoxicity kit in two cancer cell lines including SMMC7721 and EC9706. The viability results showed that complexes of protonated ellipticine could significantly reduce the viability of the two cell lines. The results described herein provide further incentives for basic studies on self-assembling peptide-based delivery of hydrophobic anticancer drugs.