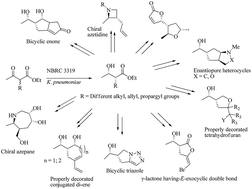
A series of structurally novel small ring carbocyclic and heterocyclic molecules were accessed in an enantiopure fashion. The starting materials, α-substituted-β-hydroxyesters, were achieved through the biocatalytic dynamic kinetic resolution of parent β-ketoesters in an excellent enantio and diastereocontrolled way. The active functional groups present in the starting precursor, were then tuned sequentially through certain key transformations (functional group interconversions; FGIs) to yield several small molecular scaffolds in a diverse way. Specific transformations such as halocyclization, ene–yne metathesis, dipolar cycloaddition, Mitsunobu cyclization, ring closing metathesis and Pauson–Khand reactions were mainly applied to generate the diversity.