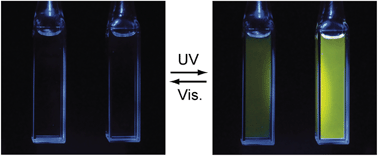
The sulfone derivatives of 1,2-bis(2-alkyl-6-phenyl-1-benzothiophen-3-yl)perfluorocyclopentene having various short alkyl chain substituents at reactive carbons were prepared and the effect of alkyl substitution on the fluorescence property of the closed-ring isomers was studied. Upon irradiation with ultraviolet (UV) light the derivatives exhibit a brilliant green fluorescence under irradiation with visible (> 400 nm) light, while the fluorescence disappears upon irradiation with visible (> 400 nm) light alone. The fluorescence quantum yield of the methyl substituted derivative (1b) dramatically decreases from 0.84 to 0.15 when the solvent is changed from hexane to acetonitrile, while the changes of ethyl, n-propyl and n-butyl substituted derivatives (2b)–(4b) are moderate. The quantum yields of (2b)–(4b) are kept to values close to 0.7 even in polar acetonitrile. The fluorescence lifetime measurement revealed that efficient non-radiative decay processes took place in (1b) in polar solvent, while their contribution to the deactivation was not so large in (2b)–(4b). The neighboring short alkyl chains at the connecting carbons are considered to defend the sulfone units against the attack of polar solvent molecules and weaken the solvent polarity effect.