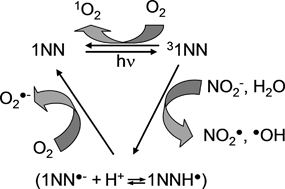
The excited triplet state of 1-nitronaphthalene, 1NN, (31NN) is able to oxidise nitrite to ˙NO2, with a second-order rate constant that varies from (3.56 ± 0.11) × 108 M−1 s−1 (μ ± σ) at pH 2.0 to (3.36 ± 0.28) × 109 M−1 s−1 at pH 6.5. The polychromatic quantum yield of ˙NO2 photogeneration by 1NN in neutral solution is Φ˙NO21NN ≥ (5.7 ± 1.5) × 107 × [NO2−]/{(3.4 ± 0.3) × 109 × [NO2−] + 6.0 × 105} in the wavelength interval of 300–440 nm. Irradiated 1NN is also able to produce ˙OH, with a polychromatic quantum yield Φ˙OH1NN = (3.42 ± 0.42) × 10−4. In the presence of 1NN and NO2−/HNO2 under irradiation, excited 1NN (probably its triplet state) would react with ˙NO2 to yield two dinitronaphthalene isomers, 15DNN and 18DNN. The photonitration of 1NN is maximum around pH 3.5. At higher pH the formation rate of ˙NO2 by photolysis of NO2−/HNO2 would be lower, because the photolysis of nitrite is less efficient than that of HNO2. At lower pH, the reaction between 31NN and ˙NO2 is probably replaced by other processes (involving e.g.31NN-H+) that do not yield the dinitronaphthalenes.