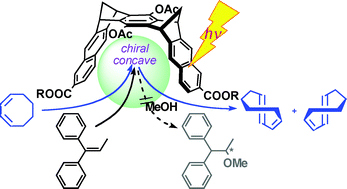
Inherently chiral molecular clip (MC) 2 binds (Z,Z)-1,3-cyclooctadiene (COD) and 1,1-diphenylpropene (DPP) in 4:1:5 THF-MeOH-H2O solution (at 25 °C) with association constants of 8800 and 27000 M−1, respectively. The thermodynamic parameters obtained from the van't Hoff analysis (ΔH° = −96.4 kJ mol−1, ΔS° = −239 J mol−1K−1) reveal that the binding of DPP by MC is strongly driven by the enthalpic gain from hydrophobic and π–π stacking interactions, which is however largely cancelled out by the entropic loss arising from the tight molecular association. Supramolecular photosensitization by MC 2 facilitates the Z–Eisomerization of COD to chiral (E,Z)-isomer in a good E/Z ratio of 0.19 and a low ee of 0.7%, but does not appear to work with DPP probably due to the less-efficient electron transfer in the acceptor–donor–acceptor complex of DPP with MC 2.