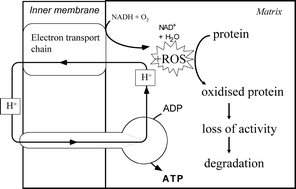
Plant mitochondria produce reactive oxygen species (ROS) as an unavoidable side product of aerobic metabolism, but they have mechanisms for regulating this production such as the alternative oxidase. Once produced, ROS can be removed by several different enzyme systems. Finally, should the first two strategies fail, the ROS produced can act as a signal to the rest of the cell and/or cause damage to DNA, lipids and proteins. Proteins are modified in a variety of ways by ROS, some direct, others indirect e.g. by conjugation with breakdown products of fatty acid peroxidation. Reversible oxidation of cysteine and methionine side chains is an important mechanism for regulating enzyme activity. Mitochondria from both mammalian and plant tissues contain a number of oxidised proteins, but the relative abundance of these post-translationally modified forms is as yet unknown, as are the consequences of the modification for the properties and turnover time of the proteins. Specific proteins appear to be particularly vulnerable to oxidative carbonylation in the matrix of plant mitochondria; these include several enzymes of the Krebs cycle, glycine decarboxylase, superoxide dismutase and heat shock proteins. Plant mitochondria contain a number of different proteases, but their role in removing oxidatively damaged proteins is, as yet, unclear.