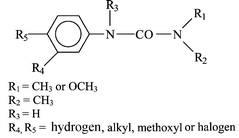
The photochemical behaviour of phenylurea herbicides in aqueous solution is highly dependent on the nature and position of substituents on the ring. Most of these herbicides are methylated on the urea moiety, the other substituents are usually halogens or methoxy groups. The main reaction involving the aromatic ring of unhalogenated phenylureas excited at wavelengths shorter than 300 nm is an intramolecular rearrangement, similar to photo-Fries rearrangement, whereas with halogenated derivatives, photohydrolysis is the main transformation pathway. In the particular case of para-halogenated phenylureas, the intermediate formation of a carbene is observed. When the urea moiety is substituted with a methoxyl group, demethoxylation is a competitive reaction. N-Demethylation or oxidation of methyl groups is also observed, but with a lower yield. Photooxidation of phenylureas can also be induced by photocatalysis, iron salts or humic substances. In the absence of water, the main route for phototransformation of diuron is the oxidation or elimination of methyl groups. It is entirely possible that a photochemical intermediate could turn out to be more toxic than the initial herbicide.