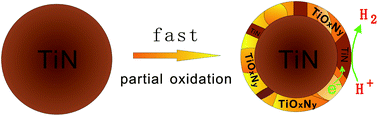
The non-equilibrium quick oxidation of titanium nitride (TiN) particles is found to be capable of kinetically controlling the partial oxidation on the surface and constructing a TiOxNy@TiN core–shell structure, in which the TiOxNy shell exhibits different components (x = 0.0–2.0). The TiOxNy@TiN structure can absorb visible light and display a high photocatalytic activity for hydrogen production, without any help from noble metal co-catalysts. A hydrogen generation rate of 506 μmol g−1 h−1 can be reached. The strong catalytic ability of TiOxNy@TiN can be attributed to the formation of heterojunction among the hybrid TiOxNy, which can facilitate the separation of light-induced electron–hole pairs. Furthermore, the non-equilibrium quick oxidation leads to the partial survival of electro-conductive TiN tiny crystals on the surface, which can act as co-catalysts to further improve the electron–hole separation and to catalyze hydrogen ion reduction.