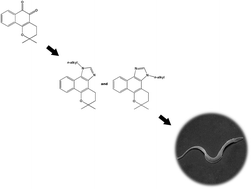
The QSAR study of 34 2-aryl-naphthoimidazoles screened so far revealed that σi is the most important factor for their lytic activity on the bloodstream trypomastigote forms of T. cruzi, the etiologic agent of Chagas disease. Based on this result, 16 new N-alkyl-naphthoimidazoles derived from 6,6-dimethyl-3,4,5,6-tetrahydrobenzo[7,8]chromene[5,6-d]imidazole (the product of the reaction of β-lapachone with paraformaldehyde) by its reaction with halo-alkanes were prepared and evaluated against the parasite and peritoneal macrophages. The N1-n-hexyl and N3-n-hexyl naphthoimidazoles were 2.2 and 3.2 times more active than the standard drug benznidazole with selectivity indices of 2.7 and 13.4, respectively.