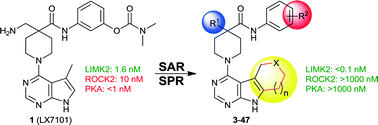
Extensive structure–activity studies on three different modification sites resulted in a series of LIM kinase inhibitors, containing a novel tricyclic hinge-binding motif based on the pyrrolopyrimidine scaffold. The compounds display a superior selectivity profile and significantly increased on-target activity compared to the former clinical candidate LX7101 (Lexicon Pharmaceuticals). Additionally, a soft drug approach to yield locally active analogues was successfully implemented.