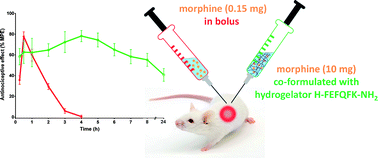
Herein, a family of hydrogel-forming peptides was designed starting from the short, tunable and amphipathic hexapeptide hydrogelator H-Phe-Glu-Phe-Gln-Phe-Lys-OH (1). The hydrophobic side chains as well as the nature of both N- and C-termini were modified in order to obtain suitable gelation conditions and drug release profiles for in vivo application. To potentially increase the enzymatic stability, an all-D analogue was prepared as well. After their macroscopic and microscopic characterization by rheology and transmission electron microscopy (TEM) analysis, ****** drugs were encapsulated into the hydrogels and sustained release experiments were carried out. Hydrogel toxicity was assessed in cell viability assays. Based on the physicochemical, mechanical, and noncytotoxic properties, H-Phe-Glu-Phe-Gln-Phe-Lys-NH2 (2) was further investigated for in vivo release of morphine. The antinociceptive effects following subcutaneous injection of the morphine-containing hydrogel 2 was evaluated in a model of thermal nociception using the mouse tail-flick test. Sustained antinociceptive effects over extended periods of time (up to 24 h) for morphine co-formulated with hydrogel 2, compared to morphine injection in solution (effects up to 2 h), were observed.