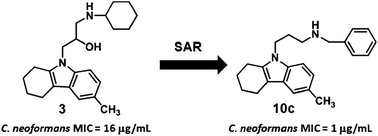
A Saccharomyces cerevisiae N-myristoyltransferase (NMT) inhibitor bearing a tetrahydrocarbazole scaffold was found to possess broad-spectrum antifungal activity. A series of C6- and N9-modified tetrahydrocarbazole derivatives were designed and synthesized. An in vitro antifungal assay indicated that several tetrahydrocarbazole derivatives showed improved activity with a broad spectrum. Particularly, the inhibitory activity of compound 10c against Cryptococcus neoformans, Aspergillus fumigatus and M. gypseum was comparable or superior to that of fluconazole and benzofuran NMT inhibitors. The present study provides a good starting point for the discovery of novel antifungal agents.