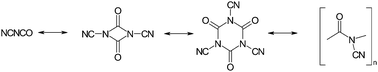
The first inorganic polyisocyanate, (NC–NCO)x, has been prepared and investigated. By polyaddition (polymerisation) of the reactive monomer cyanogen isocyanate (NC–NCO) at low temperatures, the moisture-sensitive polymer was obtained as a phase pure solid. Using several analytical techniques (UV, IR, NMR, MS, DSC, TGA), the formation and the principal connectivity patterns of the hydrogen-free amorphous inorganic network have been settled. From the experimental results, a close structural relation to the organic “nylons” was deduced. Upon thermal impact, this inorganic macromolecule behaves like a typical thermosetting material. Exposing polymeric C2N2O to a temperature between 400 and 500 °C leads to quantitative removal of oxygen in the form of CO2. Depolymerisation and fragmentation at higher temperatures resulted in a virtually complete mass loss due to release of gaseous species. Some preliminary results concerning the high-pressure transformation towards a crystalline “C2N2O” (carbon oxynitride) are also given.