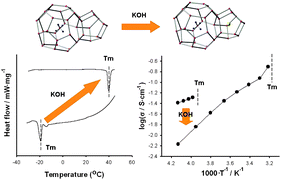
The hydroxide clathrate hydrates exhibit high ionic conductivity even at quite low temperature because of the relatively high mobility of OH− ions in the water host framework. The unique crystalline structure of Me4NOH·10H2O facilitates a transportation of charge carriers, but its low melting temperature of −20 °C decisively hinders practical applications. We confirmed that the co-inclusion of a secondary guest, using KOH in the present study, in Me4NOH clathrate hydrate can induce a drastic increase of the melting temperature from −19.1 °C to +39.8 °C without any occurrence of structural distortion. For this ionic clathrate hydrate the ionic conductivity reaches the value of 0.20 S·cm−1, suggesting a potential super-ionic conductor. For real applications to ionic conductors the electrochemical stability region was checked by cyclic voltammetry (CV) and it was found that Me4NOH·9H2O·KOH showed a wide electrochemical window corresponding to 5.8 V. To synthesize a hydrate-based proton conductor with acceptable thermal stability and electrochemical properties, the specific anionic and cationic species have to be examined with the full consideration of the structural framework and anion/proton migration.