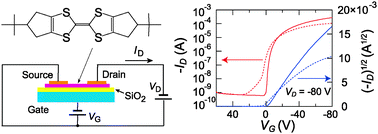
Hexamethylenetetrathiafulvalene (HMTTF) derivatives substituted by tert-butyl, n-pentyl, and 1,1-dimethylpropyl groups are prepared, and the transistor properties are investigated. The compounds substituted by bulky tertiary groups exhibit high mobilities up to 0.98 cm2/Vs in the thin-film transistors and 2.3 cm2/Vs in the single-crystal transistors. At the same time these compounds realize low threshold voltages close to zero and large on–off ratios. The high mobility and the low threshold voltage are maintained more than one month in air. The single-crystal X-ray structure analyses reveal uniform stacking structures. These observations demonstrate that the low threshold voltage and the stable device performance are not solely determined by the energy levels, but are remarkably improved by the closely packed structures derived from the bulky alkyl groups.