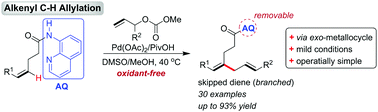
An alkenyl C–H allylation by an exo-palladacycle intermediate is demonstrated, employing unactivated (Z)-alkenes and allyl carbonates. With the use of an 8-aminoquinoline (AQ) derived amide as the directing group, the N,N-bidentate-chelation-assisted C–H activation protocol proceeded under mild and oxidant-free conditions with excellent selectivity. The utility of this approach is demonstrated by the preparative scale, selective conversion of inseparable Z/E alkenes and ready removal of the amide auxiliary to provide the corresponding ester.