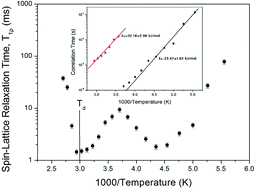
In order to understand the thermodynamic properties and structural geometry of NH4Al(SO4)2·12H2O, we studied the magic angle spinning nuclear magnetic resonance (MAS NMR) and static NMR as a function of temperature. The changes of the chemical shifts, linewidths, resonance frequencies, and spin–lattice relaxation times for 1H, 14N, and 27Al nuclei near Td (=335 K), which is interpreted as the onset of partial thermal decomposition, are due to the structural phase transition. The changes near Td are related to the loss of H2O, which probably disrupts the forms of the octahedra of water molecules surrounding NH4+ and Al3+ ions. This mechanism above Td is related to hydrogen-bond transfer involving the breakage of the weak part of the hydrogen bond.