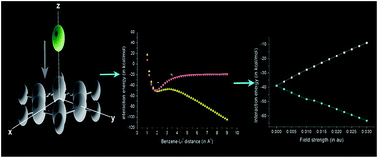
The effect of an external electric field (EEF) on cation–pi interaction between benzene and alkali metal ions has been studied using density functional (DFT) theory and MP2 calculations. Results confirmed that the interaction energy and reactivity of the complexes are sensitive towards the strength as well as direction of the EEF. When EEF is applied perpendicularly to the benzene ring, a linear variation of interaction energy with the field strength is observed. It is further observed that the EEF imparts a significant impact on the curvature of potential energy surface (PES) bringing about a modification on interaction energy. Similarly, the distance of the cation from the pi-ring is also affected by EEF. Results show an inverse dependence of cation–pi distance on the strength of the EEF.