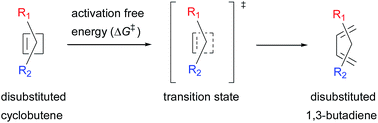
The captodative substitution effect towards 4π electrocyclic ring-opening of substituted cyclobutenes is investigated using the B3LYP/6-31+G(d) method. For most of the cases, the activation free energy for the ring-opening of substituted cyclobutenes is lower than for the unsubstituted cyclobutenes and for several captodative substitution patterns, it differs by more than 15.0 kcal mol−1. The lowest activation energy barrier is computed for trans-3-C(O)CH3–4-NH2 substituted cyclobutene, which is remarkably 23.7 kcal mol−1 lower than for the unsubstituted cyclobutene. In addition to this, an extra acceleration effect exerted by the captodative substitution is probed through energetic analyses.