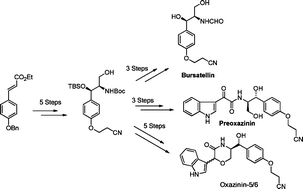
Asymmetric first total syntheses of the unprecedented toxins oxazinin-5, oxazinin-6 and preoxazinin-7 have been achieved from a common key intermediate 18, derived from a regiocontrolled Sharpless asymmetric aminohydroxylation and oxa-Michael reaction, which in addition to confirming the structure also established the absolute configuration of the natural products. On the way an expeditious synthesis of a metabolite bursatellin was completed in 8 steps.