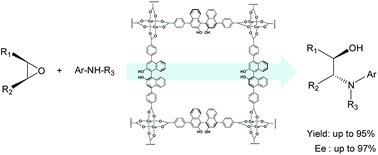
An efficient asymmetric ring-opening (ARO) reaction of meso-epoxides with aromatic amines catalysed by a series of homochiral metal–organic frameworks (MOFs) was carried out. Excellent results (up to 95% ee) for the ARO of cyclohexene oxide with several aromatic amines were achieved with a homochiral MOF derived from the ligand (R)-2,2′-dihydroxyl-1,1′-binaphthalene-5,5′-dicarboxylic acid. Furthermore, homochiral MOFs based on (R)-2,2′-dihydroxy-1,1′-binaphthyl-4,4′-di(4-benzoic acid) and (R)-2,2′-diethoxy-1,1′-binaphthyl-4,4′-di(5-isophthalic acid) catalysed ARO reactions of cis-stilbene oxide with 1-naphthylamine in high yield (up to 95%) and excellent enantioselectivity (up to 97%) of the β-amino alcohol. The MOF catalysts were recoverable and recyclable with retention of their performance.