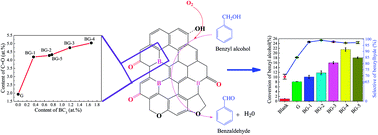
Boron-doped graphene samples (BGs) with tunable boron content of 0–2.90 at% were synthesized and directly used in the gas-phase oxidation of benzyl alcohol to benzaldehyde, and showed excellent performance. XPS results indicated that the graphitic sp2 B species (BC3) is the mainly boron dopant species incorporated in the graphene lattice, which could significantly improve the content of ketone carbonyl groups (C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O) on the graphene. For instance, the contents of C
O) on the graphene. For instance, the contents of C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O jumped from 1.93 to 4.19 at% while BC3 doped into the graphene lattice was only 0.35 at%. The C
O jumped from 1.93 to 4.19 at% while BC3 doped into the graphene lattice was only 0.35 at%. The C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) O is the active site of catalytic reaction, so BG has significantly improved catalytic activity. Compared to the un-doped graphene (G), the conversion of benzyl alcohol over BGs increased 2.35 times and the selectivity of benzaldehyde increased from 77.3% to 99.2%. Aerobic–anaerobic exchange experiments revealed that the superior catalytic performance of BG was achieved only under aerobic conditions. The study of the boron-doped carbocatalyst may also provide guidance for the design of surface modified carbon-based catalysts for the selective oxidation dehydrogenation of alcohols by regulating doping elements and their types.
O is the active site of catalytic reaction, so BG has significantly improved catalytic activity. Compared to the un-doped graphene (G), the conversion of benzyl alcohol over BGs increased 2.35 times and the selectivity of benzaldehyde increased from 77.3% to 99.2%. Aerobic–anaerobic exchange experiments revealed that the superior catalytic performance of BG was achieved only under aerobic conditions. The study of the boron-doped carbocatalyst may also provide guidance for the design of surface modified carbon-based catalysts for the selective oxidation dehydrogenation of alcohols by regulating doping elements and their types.