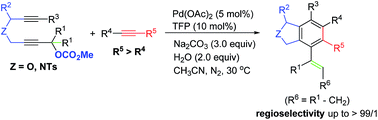
A palladium-catalyzed highly regio- and chemo-selective cyclization of 2,7-alkadiynylic carbonates with functionalized alkynes to construct 1,3-dihydroisobenzofuran and isoindoline derivatives under mild conditions has been developed. Functional groups such as alcohol, sulfonamide, and indoles could be well tolerated. After careful mechanistic studies, a mechanism involving oxidative addition and regioselectivity-defined double alkyne insertions has been proposed.