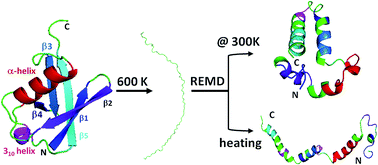
The folding of peptides into three-dimensional structures has been correlated to the occurrence of biological phenomena. In order to fully understand the pathway of protein folding, the structure of the denatured state, the reference state, under native conditions should be well-defined. However, very few of the denatured proteins have been studied under native conditions because the denatured state cannot be determined under exact native conditions using known experimental approaches. Herein, we characterize the denatured state ensembles (DSE) of ubiquitin under native conditions starting from a fully extended sequence resembling a peptide generated from a ribosome using replica exchange molecular dynamics. The representative structures of DSE with specific secondary structures distinct from the native ones are discussed. This result allows us to define the protein folding pathway more unambiguously and opens the door to biomedical studies of diseases correlated to protein misfolding. Potentially, the insights into the DSE can lead to targeted design of structure-based drug.