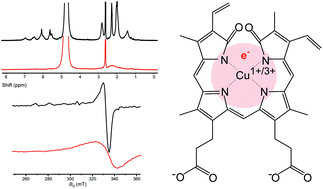
Biliverdin (BV), a product of heme catabolism, is known to interact with transition metals, but the details of such interactions under physiological conditions are scarce. Herein, we examined coordinate/redox interactions of BV with Cu2+ in phosphate buffer at pH 7.4, using spectrophotometry, HESI-MS, Raman spectroscopy, 1H NMR, EPR, fluorimetry, and electrochemical methods. BV formed a stable coordination complex with copper in 1 : 1 stoichiometry. The structure of BV was more planar and energetically stable in the complex. The complex showed strong paramagnetic effects that were attributed to an unpaired delocalized e−. The delocalized electron may come from BV or Cu2+, so the complex is formally composed either of BV radical cation and Cu1+ or of BV radical anion and Cu3+. The complex underwent oxidation only in the presence of both O2 and an excess of Cu2+, or a strong oxidizing agent, and it was resistant to reducing agents. The biological effects of the stable BV metallocomplex containing a delocalized unpaired electron should be further examined, and may provide an answer to the long-standing question of high energy investment in the catabolism of BV, which represents a relatively harmless molecule per se.