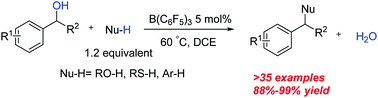An efficient and general method of nucleophilic substitution of benzylic alcohols catalyzed by non-metallic Lewis acid B(C6F5)3 was developed. The reaction could be carried out under mild conditions and more than 35 examples of ethers, thioethers and triarylmethanes were constructed in high yields. Some bioactive organic molecules were synthesized directly using the methods.