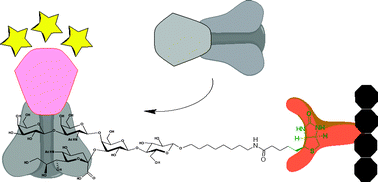
Biotinylated analogues of gangliosides GM2, GM1, GD1a and GalNAc-GD1a were synthesized in high yields using glycosyltransferases from Campylobacter jejuni. The presence of a biotin moiety in the aglycone part of these mimics allows for attachment of these materials onto various streptavidin-coated surfaces. Analysis of the interaction of biotin-appended GM1 with the B subunit of Escherichia coli heat-labile enterotoxin performed in a modified ELISA procedure shows the potential of this compound to replace the natural GM1 in toxin detection.