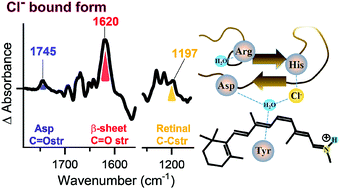
Long-wavelength-sensitive (LWS) pigment possesses a chloride binding site in its protein moiety. The binding of chloride alters the absorption spectra of LWS; this is known as the chloride effect. Although the two amino acid substitutions of His197 and Lys200 influence the chloride effect, the molecular mechanism of chloride binding, which underlies the spectral tuning, has yet to be clarified. In this study, we applied ATR-FTIR spectroscopy to monkey green (MG) pigment to gain structural information of the chloride binding site. The results suggest that chloride binding stabilizes the β-sheet structure on the extracellular side loop with perturbation of the retinal polyene chain, promotes a hydrogen bonding exchange with the hydroxyl group of Tyr, and alters the protonation state of carboxylate. Combining with the results of the binding analyses of various anions (Br−, I− and NO3−), our findings suggest that the anion binding pocket is organized for only Cl− (or Br−) to stabilize conformation around the retinal chromophore, which is functionally relevant with absorbing long wavelength light.