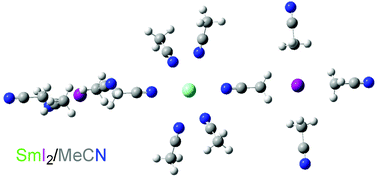
In this communication we show that the instability of samarium diiodide (SmI2) in acetonitrile is a consequence of ionization of the reductant in this solvent. Samarium triflate (Sm(OTf)2) is exceptionally stable in acetonitrile for periods over six months and can be used with appropriate additives to initiate a ketyl–olefin coupling reaction in high yield.