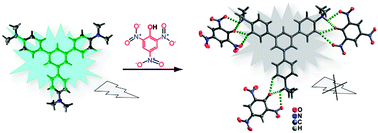
A fluorescent chemo-sensor, 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene was synthesized by substituting the N–H protons of 1,3,5-tris(4′-aminophenyl)benzene with methyl groups. The chemo-sensor shows highly selective and remarkable fluorescence quenching in the presence of picric acid with a detection limit of 1.5 ppm. The origin of the selectivity was investigated using absorption, fluorescence emission and 1H NMR spectroscopic techniques. The solid state structure of 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene and its picric acid complex reveals multiple hydrogen bonds (N–H⋯O and C–H⋯O), π–π interactions and electrostatic interactions between 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene and picric acid. The proton transfer process from picric acid to 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene results in the formation of picrate anions and the triply protonated 1,3,5-tris(4′-(N,N-dimethylamino)phenyl)benzene species containing dimethylammonium (–NHMe2+) groups.