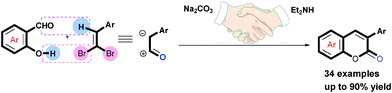
Facile synthesis of coumarin via the tandem reaction of salicylaldehyde with aryl-substituted 1,1-dibromo-1-alkene was developed. This new protocol proceeds smoothly under mild and transition-metal-free conditions, it allows rapid access to coumarins containing various heteroatoms that are more difficult to prepare by traditional methods. Based on the isolated intermediate of 4-(diethylamino)-3-phenylchroman-2-one and detailed mechanistic studies, a credible tandem pathway was proposed.