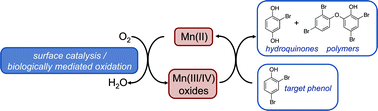
Naturally occurring manganese (Mn(III/IV)) oxides are ubiquitous in a wide range of environmental settings and play a key role in numerous biogeochemical cycles. In addition, Mn(III/IV) oxides are powerful oxidants that are capable of oxidizing a wide range of compounds. This review critically assesses the reactivity of Mn oxides with organic contaminants. Initial work with organic reductants employed high concentrations of model compounds (e.g., substituted phenols and anilines) and emphasized the reductive dissolution of the Mn oxides. Studies with lower concentrations of organic contaminants demonstrate that Mn oxides are capable of oxidizing a wide range of compounds (e.g., antibacterial agents, endocrine disruptors, and pesticides). Both model compounds and organic contaminants undergo similar reaction mechanisms on the oxide surface. The oxidation rates of organic compounds by manganese oxides are dependent upon solution conditions, such as pH and the presence of cations, anions, or dissolved organic matter. Similarly, physicochemical properties of the minerals used affect the rates of organic compound oxidation, which increase with the average oxidation state, redox potential, and specific surface area of the Mn oxides. Due to their reactivity with contaminants under environmentally relevant conditions, Mn oxides may oxidize contaminants in soils and/or be applied in water treatment applications.