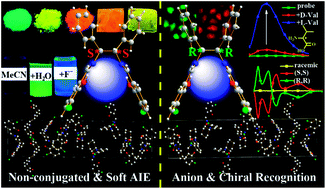
Natural products are usually non-conjugated and chiral, but organic luminescent materials are commonly polycyclic aromatic molecules with extended π-conjugation. In the present work, we combine with the advantages of non-conjugation and chirality to prepare a series of novel and simple salen ligands (41 samples), which have a non-conjugated and chiral (S,S) and (R,R) cyclohexane or 1,2-diphenylethane bridge but display strong blue, green, and red aggregation-induced emission (AIE) with large Stokes shifts (up to 186 nm) and high fluorescence quantum yields (up to 0.35). Through hydrogen and halogen bonds, these flexible salen ligands can be used as universal anion probes and chiral receptors of unprotected amino acids (enantiomeric selectivity up to 0.11) with fluorescence quantum yields up to 0.29 and 0.27, respectively. Moreover, the effects of different chiral bridges on the molecule arrangement, AIE, and anion and chiral recognition properties are also explored, which provide unequivocal insights for the design of non-conjugated chiral and soft fluorescent materials.