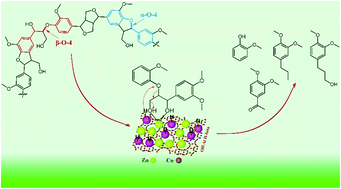
Efficient cleavage of aryl–ether linkages is a key strategy for generating aromatic chemicals and fuels from lignin. Currently, a popular method to depolymerize native/technical lignin employs a combination of Lewis acid and hydrogenation metal. However, a clear mechanistic understanding of the process is lacking. Thus, a more thorough understanding of the mechanism of lignin depolymerization in this system is essential. Herein, we propose a detailed mechanistic study conducted with lignin model compounds (LMC) via a synergistic Co–Zn/Off-Al H-beta catalyst that mirrors the hydrogenolysis process of lignin. The results suggest that the main reaction paths for the phenolic dimers exhibiting α-O-4 and β-O-4 ether linkages are the cleavage of aryl–ether linkages. Particularly, the conversion was readily completed using a Co–Zn/Off-Al H-beta catalyst, but 40% of α-O-4 was converted and β-O-4 did not react in the absence of a catalyst under the same conditions. In addition, it was found that the presence of hydroxyl groups on the side chain, commonly found in native lignin, greatly promotes the cleavage of aryl–ether linkages activated by Zn Lewis acid, which was attributed to the adsorption between Zn and the hydroxyl group. Followed by the cobalt catalyzed hydrogenation reaction, the phenolic dimers are degraded into monomers that maintain aromaticity.