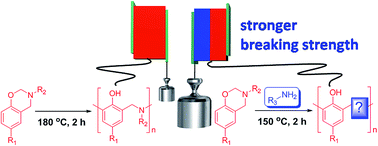
Five commercially available amines: m-phenylenediamine (A1), m-xylylenediamine (A2), isophorone diamine (A3), trimethylhexamethylenediamine (A4), and 4,4′-diaminodiphenyl sulfone (A5), were examined as nucleophilic hardeners for bis-benzoxazine monomers based on aniline paired with bisphenol-A (BA-a) or bisphenol-F (BF-a). The reactivities and reaction mechanisms of their mixtures with BA-a were investigated using FTIR and NMR spectroscopy, DSC, and HPLC techniques. It was found that BA-a rapidly cured with the amines upon heating at 120 °C or 150 °C. The cure rate was similar to the amine/epoxy curing process in practical uses, and significantly faster than the ring-opening polymerization of bulk BA-a. The possible reaction mechanism was supported by the experimental results and includes three successive steps: (i) nucleophilic substitution at the carbon atom (O–C–N) in the oxazine ring by the amine, (ii) thermal decomposition of the resulting aminomethanaminium structure, and (iii) electrophilic addition of the newly formed iminium ion with the aromatic ring to form stable aminomethylphenol structures. These findings are helpful to improve the thermosetting resins in terms of their chemical structure, material properties, and processability.