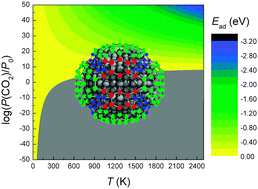
The use of carbon nanostructures to capture and store waste carbon, such as methane and carbon dioxide, is intrinsically attractive, particularly if the same molecules can be subsequently used as synthetic precursors. However, to facilitate adsorption of these highly stable species high pressures are required, and fragile carbon-based nanostructures may not survive. By combining electronic structure simulations and ab initio thermodynamics, we have investigated the thermochemical conditions required to adsorb CH, CH2, CO and CO2 on diamond nanoparticles, which can withstand higher temperatures and pressures than alternative carbon-based nanostructures. We find that, while CO2 must be over-saturated to facilitate stable adsorption (with high efficiency), the strength of the resultant C–O bonds means that desorption will not occur spontaneously when atmospheric pressure is resumed.