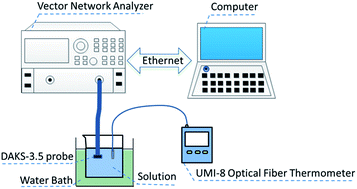
The effective permittivity of a pyridine (C5H5N)–ethanol (C2H5OH) mixed solution has been measured over the whole range of concentrations at 293.15, 303.15 and 313.15 K. Surprisingly, an exceptional phenomenon is found that the real part ε′r and the imaginary part ε′′r of the mixed solution permittivity are larger than those of their pure components at certain concentrations. To clearly understand the exceptional behavior of the dielectric spectrum, a systematic investigation on the molecular clusters ((C5H5N)m(C2H5OH)n (m = 0–4; n = 0–6)) as well as the dipole moments and cluster radii of the molecular clusters has been performed using density functional theory (DFT) calculations. Comparing the dipole moments and cluster radii of the binary clusters ((C5H5N)m(C2H5OH)n) with those of the pure clusters ((C5H5N)m and (C2H5OH)n), the results have given a reasonable explanation of the unusual behaviors of the real part ε′r and the imaginary part ε′′r of the mixed solution permittivity.