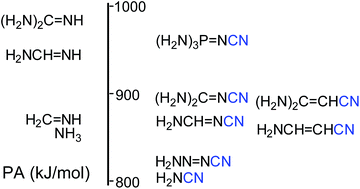
Effects of the pushing groups (electron donors) for nitriles increase as follows: H2N < H2N–N![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N < H2N–CH
N < H2N–CH![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) CH < H2N–CH
CH < H2N–CH![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N < (H2N)2C
N < (H2N)2C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) CH < (H2N)2C
CH < (H2N)2C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N < (H2N)3P
N < (H2N)3P![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N. The G2(MP2)-calculated PA(N-cyano) for (H2N)2C
N. The G2(MP2)-calculated PA(N-cyano) for (H2N)2C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N–C
N–C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) N and (H2N)3P
N and (H2N)3P![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) N–C
N–C![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) N are larger than that of HC
N are larger than that of HC![[triple bond, length as m-dash]](http://www.rsc.org/images/entities/char_e002.gif) N by 186 and 250 kJ mol−1, respectively. The hypothesis of protonation in the gas phase at the N-imino and N-amino atoms, corresponding respectively to PAs weaker by 30 and 70 kJ mol−1 than that of the N-cyano site, can be rejected.
N by 186 and 250 kJ mol−1, respectively. The hypothesis of protonation in the gas phase at the N-imino and N-amino atoms, corresponding respectively to PAs weaker by 30 and 70 kJ mol−1 than that of the N-cyano site, can be rejected.