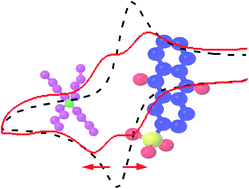
The electrode potentials of quinone redox centres in aqueous solutions can be tuned by varying the electrolyte cation identity. The phenomenon is due to the ion pairing effect of the tetra-n-butylammonium cation with the semiquinone intermediate species.