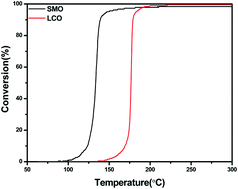
Mullite oxide SmMn2O5 (SMO) was synthesized through a co-precipitation method. The CO + O2 model system and more realistic gas mixtures were evaluated, and compared with perovskite LaCoO3 (LCO), SMO showed a better reaction activity as well as a higher catalytic activity and stronger hydrothermal resistance. Characterizations including XRD, BET, SEM, XPS, H2-TPR, O2-TPD, and CO + O2 pulse were undertaken in order to gain insight into the origin of the SMO three-way catalytic activity. The lower adsorbed oxygen reduction temperature for SMO by H2-TPR indicated a better reducibility. O2-TPD suggested that SMO exhibited excellent mobility of β-oxygen species. CO + O2 pulse further revealed that the SMO catalyst had a better oxygen storage capacity than the LCO perovskite did. With a better reducibility, facile mobility of oxygen species and better oxygen storage capacity, mullite oxide SMO showed a preferable three-way catalytic activity.