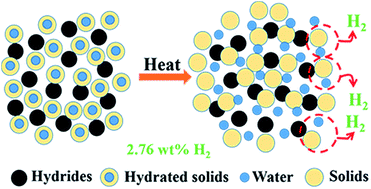
The reaction of water with hydrides (hydrolysis reaction) is very attractive for onsite hydrogen generation. Instead of using liquid water like in most cases, we demonstrate that hydrogen generation from hydrolysis reactions can also occur in the solid state. By simply heating mixtures of hydrated solids and hydrides, hydrogen generation is readily achieved through the recombination from the protonic hydrogen in the hydrated solids and the hydridic hydrogen in the hydride. The composites 5CaH2 + Na4P2O7·10H2O and NaBH4 + H2C2O4·2H2O give attainable gravimetric hydrogen storage capacity of 2.76% at 40 °C and 2.79% at 70 °C with rapid response, respectively. The dehydrogenation temperature can be controlled by the dehydration temperature of the hydrated solids. This innovative hydrogen generation approach provides a temperature activated, easy to control solution for onsite hydrogen generation.