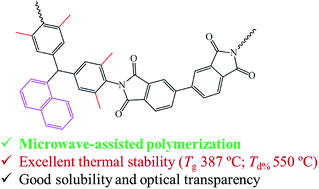
Three kinds of naphthalene-containing diamines with –H, –CH3 or –CH(CH3)2 substituents at the ortho-positions of the aniline ring including 4,4′-(naphthalen-1-ylmethylene)dianiline (BAN-1), 4,4′-(naphthalen-1-ylmethylene)bis(2,6-dimethylaniline) (BAN-2) and 4,4′-(naphthalen-1-ylmethylene)bis(2,6-diisopropylaniline) (BAN-3) were synthesized via a simple one-step electrophilic substitution reaction. These diamines were then reacted with three commercial dianhydrides, via chemical imidization under microwave irradiation, to obtain nine types of polyimide (PI). It was found that the introduction of alkyl side groups can improve the solubility and optical transparency of PIs. Moreover, compared with BAN-2 based PIs containing –CH3 groups, BAN-3 based PIs containing –CH(CH3)2 groups exhibited better solubility and optical transparency (transmittances at 450 nm of over 86%). Meanwhile, due to the presence of the rigid naphthalene side groups, all the PIs possessed high thermal stability with a glass transition temperature (Tg) of over 290 °C and a decomposition temperature at 5% weight loss of over 510 °C under nitrogen. Furthermore, the Tg of PI-2B composed of BAN-2 and 3,3′,4,4′-biphenyltetracarboxylic dianhydride (BPDA) was found to be as high as 387 °C, which is comparable to that of the commercial and conventional PI material (Kapton®, Tg = 390 °C).