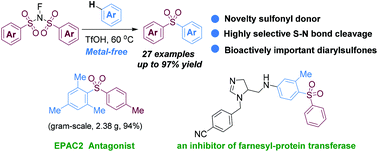
A novel metal-free sulfonylation of arenes with N-fluorobenzenesulfonimide (NFSI) toward the synthesis of diarylsulfones has been developed. The reaction represents a rare example of sulfonylation reaction using NFSI as an efficient sulfonyl donor and the first example of acid-mediated sulfonylation of unactivated arenes with NFSI via selective cleavage of S–N bonds. This protocol provides a concise approach for the construction of pharmaceutically and biologically important diarylsulfones. Applications in the functionalization of natural products (e.g., β-estradiol) and in the synthesis of a key intermediate to an inhibitor of farnesyl-protein transferase, as well as in the gram-scale synthesis of the EPAC2 antagonist, are demonstrated.