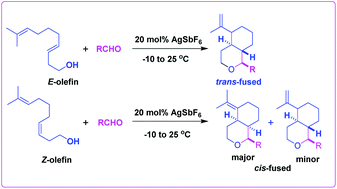
E- and Z-9-Methyldeca-3,8-dien-1-ols undergo smooth cyclization with aldehydes in the presence of 20 mol% AgSbF6 under extremely mild conditions to generate the corresponding oxa-bicycles in good yields with excellent selectivity. In fact, E-olefin affords the trans-product exclusively, whereas the Z-olefin gives the cis-product predominantly. In the case of E- or Z-8-methylnona-3,8-dien-1-ol, the product is formed via the termination of Prins cyclization with an allylic C–H bond through olefin migration. The termination of Prins cyclization with tethered olefin is an unprecedented reaction, which provides a useful motif of various natural products.