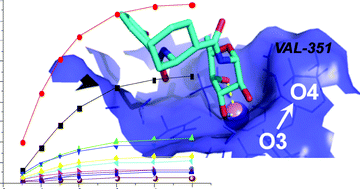
In this work, we have studied in detail the binding of two α-fucosylamide-based mimics of LewisX to DC-SIGN ECD (ECD = extracellular domain) using STD NMR and docking. We have concluded that the binding mode occurs mainly through the fucose moiety, in the same way as LewisX. Similarly to other mimics containing mannose or fucose previously studied, we have shown that both compounds bind to DC-SIGN ECD in a multimodal fashion. In this case, the main contact is the interaction of two hydroxyl groups one equatorial and the other one axial (O3 and O4) of the fucose with the Ca2+ as LewisX and similarly to mannose-containing mimics (in this case the interacting groups are both in the equatorial position). Finally, we have measured the KD of one mimic that was 0.4 mM. Competitive STD NMR experiments indicate that the aromatic moiety provides additional binding contacts that increase the affinity.