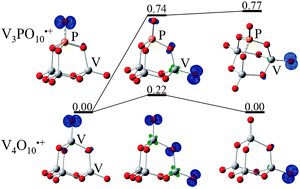
A series of vanadium and phosphorus heteronuclear oxide cluster cations (VxPyOz+) are prepared by laser ablation and the reactions of V3PO10˙+ and V4O10˙+ with methane in a fast flow reactor under the same conditions are studied. A time of flight mass spectrometer is used to detect the cluster distribution before and after reactions. In addition to previously identified reaction of V4O10˙+ + CH4 → V4O10H+ + CH3˙, the observation of hydrogen atom pickup cluster V3PO10H+ suggests the reaction: V3PO10˙+ + CH4 → V3PO10H+ + CH3˙. The rate of the reaction of V4O10˙+ with CH4 is approximately 2.5 times faster than that of V3PO10˙+ with CH4. Density functional theory (DFT) calculations predict that structure of V3PO10˙+ is topologically similar to that of V4O10˙+, as well as that of P4O10˙+, which is very similar to V4O10˙+ in terms of methane activation in previous studies. The facile methane activation by the homo- and hetero-nuclear oxide clusters can all be attributed to the presence of an oxygen-centered radical (O˙) in these clusters. Further theoretical study indicates that the O˙ radical (or spin density of the cluster) can transfer within the high symmetry V4O10˙+ and P4O10˙+ clusters quite easily, and CH4 molecule further enhances the rate of intra-cluster spin density transfer. In contrast, the intra-cluster spin density transfer within low symmetry V3PO10˙+ is thermodynamically forbidden. The experimentally observed reactivity difference is consistent with the theoretical consideration of the intra-cluster spin density transfer.